 |
||
|
|
||
| |||||||||||||||
|
|
 ERDC TN-DOER-C10
March 2000
8) Place eight 500-L microcentrifuge tubes on a rack and number them from 1 to 8.
Pipet about 1 mL of iso-octane into a 1.5-mL microcentrifuge tube and place it
on the rack.
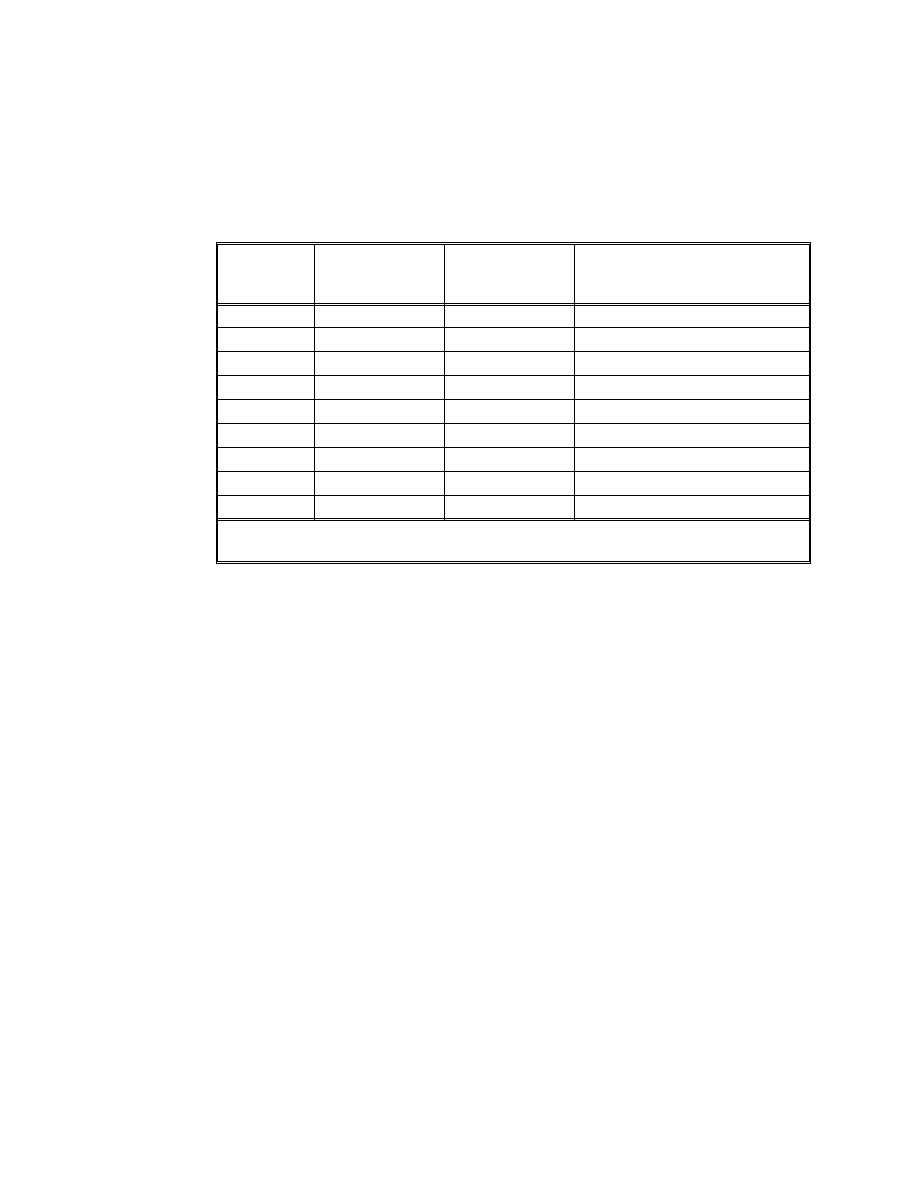
9) Prepare serial dilutions of TCDD standards using positive displacement pipettors
as follows:
Well
Tube
Concentraton,
Concentration,*
pg/mL
Makeup
pg/mL
Tube No.
TCDD Stock
1,000,000
10 L stock + 90 L of iso-octane
1
100,000
10 L tube 1 + 90 L of iso-octane
2
10,000
100
25 L tube 2 + 25 L of iso-octane
3
5,000
50
10 L tube 2 + 30 L of iso-octane
4
2,500
25
10 L tube 2 + 90 L of iso-octane
5
1,000
10
25 L tube 5 + 25 L of iso-octane
6
500
5
10 L tube 5 + 30 L of iso-octane
7
250
2.5
10 L tube 5 + 90 L of iso-octane
8
100
1
* The TCDD standards are made 100x since the cells are dosed with 2 L of the standard into
200-L cell medium.
10) Dose the cells with 2 L of iso-octane (serve as control background), TCDD
standards, and environmental samples using a positive displacement pipettor in
Plate 1, as shown on the following page
11) Dose the remaining 16 environmental samples in Plate 2 similar to that in Plate 1.
12) Write down the time and date of dosing on the lids of the plates. Dosing in the
late afternoon is preferable since the cells will be exposed to the TCDD standards
and environmental samples for 16 hours. The assay can be taken down in the
morning of the following day.
IV. DAY 4 (ASSAY TAKEDOWN)
The exposure to the TCDD standard and environmental samples is terminated at 16 hours. The
cell medium is removed, and the cells washed and lysed. The lysates are then transferred into an
opaque luminometer plate and read. The luminometer is programmed to inject two substrates
(A and B) into each well simultaneously and integrate the luminescence reading over 2 seconds.
A.
MATERIALS AND EQUIPMENT.
Multichannel motorized pipettor (250 L).
PBS, GIBCO BRL #14190-144.
Substrate A, PHARMINGEN #556868 - 1 aliquot (reconstituted).
21
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us - Support Integrated Publishing |