 |
||
|
|
||
|
Page Title:
Mechanisms and driving force (Cont.) |
||
| |||||||||||||||
|
|
 The effect of advection includes both transport by the pore water flow and
that by diffusion and dispersion. Dispersion is the additional "diffusion-like"
mixing relative to the average pore water velocity that occurs as a result of
heterogeneities in the sediments. Thus the description of advection is more
complicated than diffusion, and the model for long-term cap losses will be sub-
divided into models appropriate only when diffusion dominates and models
when both advection and diffusion/dispersion are important.
Both processes are operative only for that portion of the contaminant present
in the pore water as measured by the concentration C0. This might include con-
taminant dissolved in the pore water as well as contaminant sorbed to fine par-
ticulate or colloidal matter suspended in the pore water. The best measure of
this concentration is through direct pore water measurements. In the absence of
pore water measurements, however, linear reversible sorption can be assumed
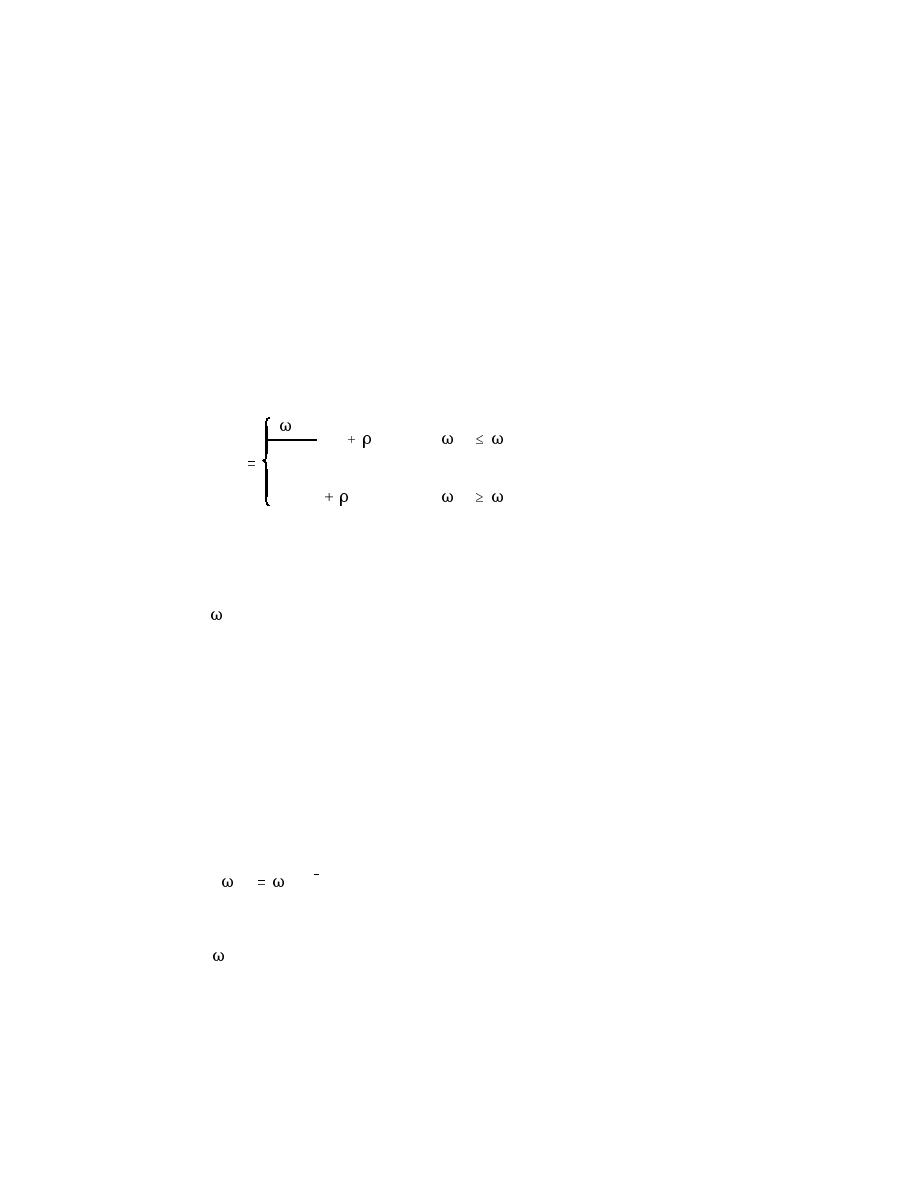
and Equations B5 or B7 apply,
sed
(1
) if
oc Koc
sed
crit
Koc foc
(B15)
C0
Cw (1
)
if
oc Koc
sed
crit
where
= sediment loading (milligrams chemical/kilogram (dry) sediment)
sed
Equation B15 indicates that the pore water concentration increases linearly with
the sediment loading until the water is saturated, that is, until the solubility limit
is reached. This limit is the normal water solubility adjusted for the sorption
onto organic matter in the pore water.
Degradation of contaminants over the long time of expected confinement is a
significant benefit of capping that should be incorporated into the design of a
cap. Polyaromatic hydrocarbons as well as chlorinated aliphatic and aromatic
compounds all exhibit slow but finite rates of degradation or transformation in
the generally anaerobic environment beneath a cap. If simple first order degra-
dation kinetics is employed, the sediment loading changes with time according to
kr t
0
(B16)
e
sed
sed
where
0
= sediment loading at time of cap placement
sed
kr = exponential time constant given by 0.693/t0.5
t0.5 = chemical half life in sediment
B8
Appendix B Model for Chemical Containment by a Cap
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us - Support Integrated Publishing |