 |
||
|
|
||
|
Page Title:
Figure D-4. Desorption isotherms for slope-derived and single-point distribution coefficients |
||
| |||||||||||||||
|
|
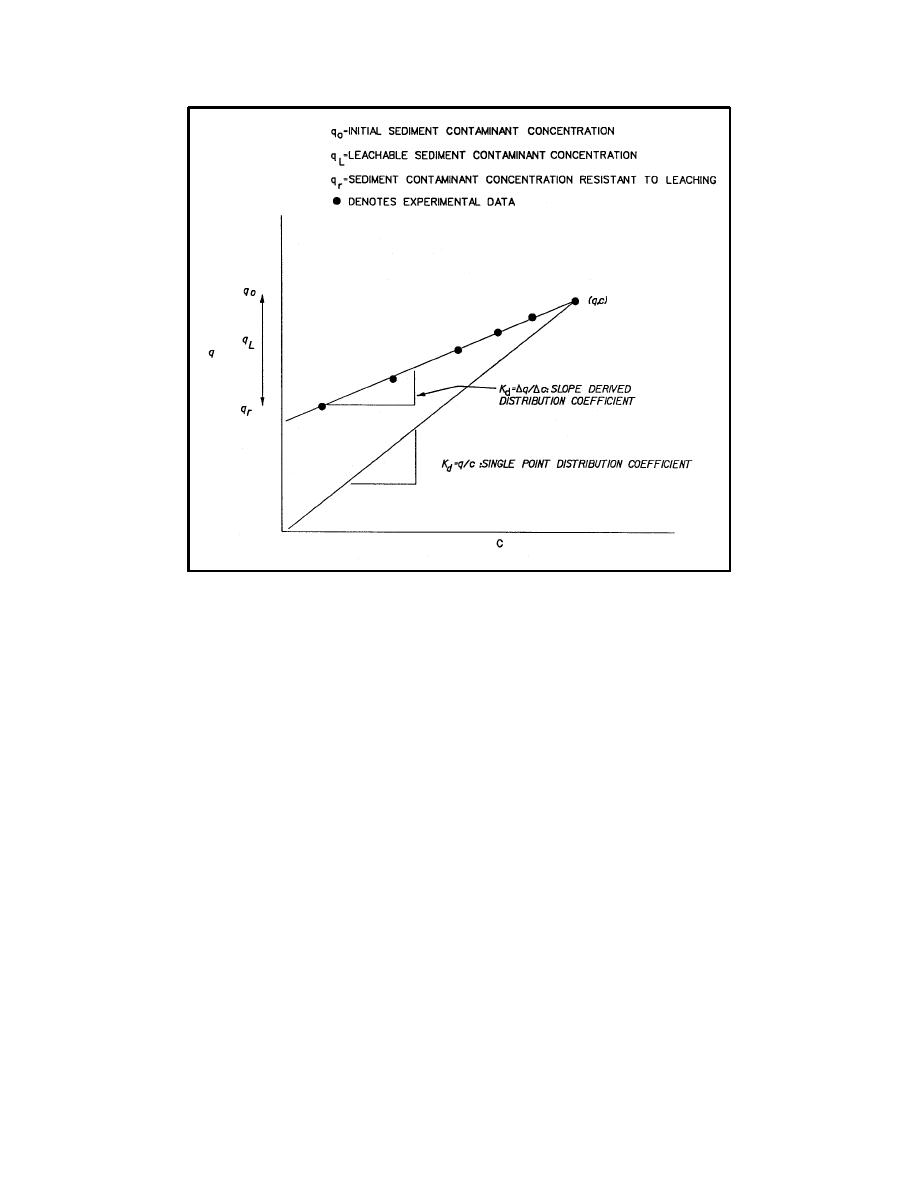 Figure D-4. Desorption isotherms for slope-derived and single-point distribution
coefficients
The general form of the q versus C relationship for classical desorption
isotherms is as follows:
q = Kd C + qr
(D-2)
where qr is contaminant concentration in solid phase resistant to leaching, mg/kg
Nonconstant distribution of contaminants between dredged material solids
and water is commonly observed during leaching of estuarine sediments
(Brannon et al. 1989; Brannon, Myers, and Price 1990; Brannon et al. 1991).
Nonconstant contaminant partitioning yields batch isotherms for which the
distribution coefficient changes as the solid phase concentration q decreases
during sequential leaching, until a turning point is reached (Figure D-5). At the
turning point, the distribution coefficient becomes constant and desorption
begins to follow the classical isotherm. The nonconstant distribution coefficient
portion of the desorption isotherm is related to elution of salt.
As salt is eluted from estuarine sediments, the ionic strength of the aqueous
phase is reduced. According to the Gouy-Chapman model of charge distribution
in double layers, decreasing the ionic strength increases repulsive forces (Stumm
and Morgan 1981) and causes the double-layer thickness between colloids to
increase. Flocculated colloidal matter becomes increasingly deflocculated and
more easily entrained in flow. The overall effect is an increase in dissolved
D6
Appendix D Leachate Testing Procedures
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us - Support Integrated Publishing |