 |
||
|
|
||
|
Page Title:
Figure D-1. Model of dredged material leaching (from Hill, Myers, and Brannon 1998) |
||
| |||||||||||||||
|
|
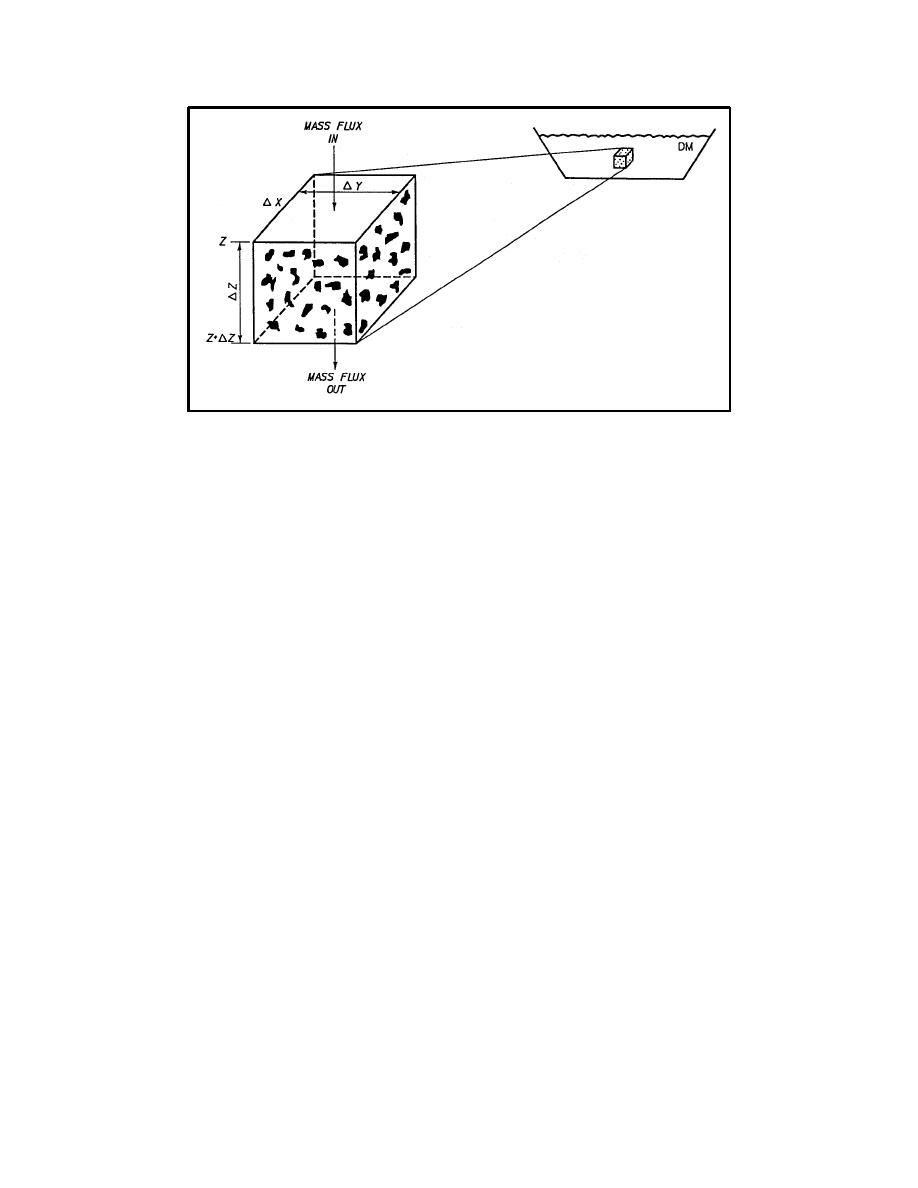 Figure D-1. Model of dredged material leaching (from Hill, Myers, and Brannon
1988)
potential just as electric current flows from a region of high electrical potential
to one of lower electrical potential. When chemical potentials are equal, the net
transfer of contaminant across the solid-water interface is zero, and the mass of
contaminant in each phase is constant, but not necessarily equal. The processes
shown in Figure D-2 control the rate at which equilibrium is reached and the
equilibrium distribution of contaminant between solid and aqueous phases.
Once equilibrium is reached, the ratio of contaminant mass in the solid phase to
the contaminant mass in the aqueous phases does not change.
In practice, a true equilibrium between dredged material solids and pore
water never exists because some of the processes shown in Figure D-2 have very
slow reaction rates. However, a pseudo steady state can be reached between
dredged material solids and water if the water is moving past the solids slowly
enough, as discussed in a following section. By assuming equilibrium between
solid and aqueous phases, the need for determining controlling processes and the
rate coefficients for these processes is eliminated. Without the equilibrium
assumption, laboratory testing and mathematical modeling would require
determination of controlling processes and investigation of the kinetics for these
processes. As is apparent from Figure D-2, predictive laboratory tests and
mathematical models based on chemical and mass transfer kinetics would be too
complicated for routine evaluation of dredged material leaching. Thus,
application of the equilibrium assumption is imperative for the development of
predictive techniques suitable for routine use.
Under equilibrium conditions, only the relative distribution of contaminant
between solid and aqueous phases is needed to predict leachate quality.
Kd = q / C
(D-1)
D2
Appendix D Leachate Testing Procedures
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us - Support Integrated Publishing |